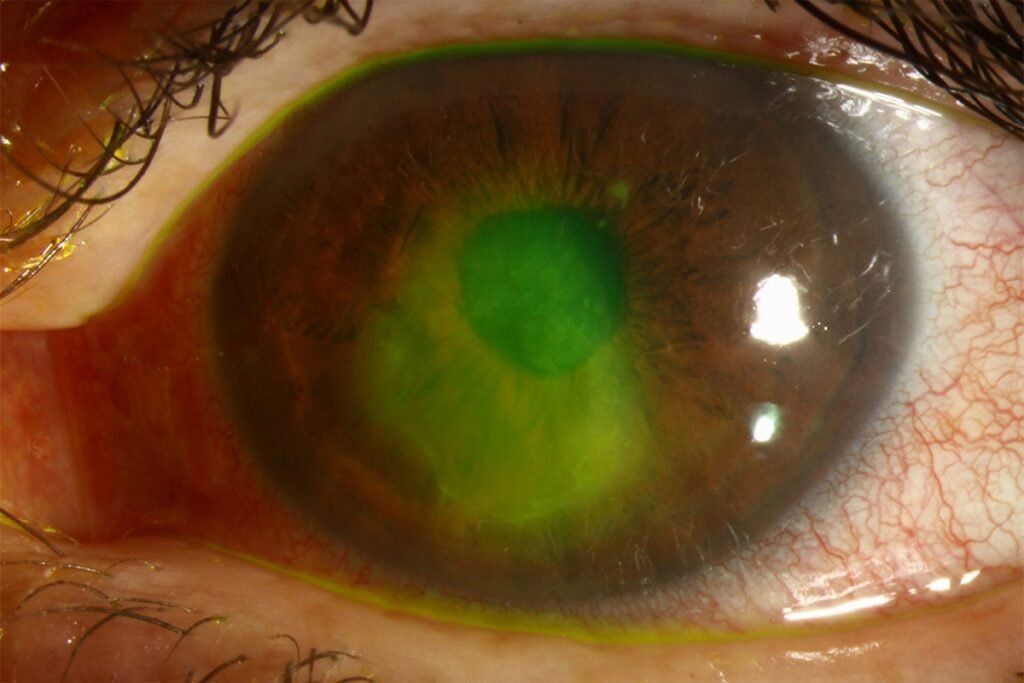
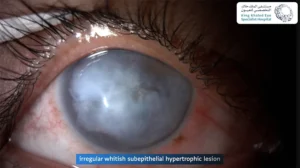
Clinical Challenge
Persistent epithelial defects (PEDs) occur when the corneal surface fails to re-epithelialize despite two weeks of standard therapy. They often arise in settings of neurotrophic keratopathy,
limbal stem cell deficiency (LSCD),post-keratoplasty, exposure keratopathy, or infection-related epithelial breakdown.Left untreated, PEDs can progress to stromal ulceration, scarring, or even perforation. This underscores the need for therapies that are both effective and accessible.
https://pubmed.ncbi.nlm.nih.gov/34093006/
What are Omnigen® and OmniLenz®?
Developed by NuVision Biotherapies (Nottingham, UK), Omnigen® is a vacuum-dried, human amniotic membrane–derived dry matrix made via the proprietary “Tereo®” process, which preserves tissue-biochemistry (growth factors, ECM) while allowing room-temperature storage.
OmniLenz® is a bespoke bandage contact lens designed to deliver Omnigen® on the ocular surface in a suture-free, outpatient setting in approximately 4-6 minutes
Omnilenz Brochure
Clinical Evidence
In a multicenter UK study (Queen Victoria Hospital & Maidstone, 84 patients / 93 PEDs):
• 58% achieved complete epithelial healing
• 28% achieved partial healing
• Mean healing time ~ 22.4 ± 12.3 days
• Efficacy across etiologies: LSCD (50% complete), microbial keratitis (57% complete)
(https://pubmed.ncbi.nlm.nih.gov/34093006/)
Another recent randomized controlled trial (NCT04553432) evaluated sutureless Omnigen® via OmniLenz® in moderate-to-severe dry eye disease (DED), showing significant symptom improvement, corneal-nerve regeneration, and anti-inflammatory effects. https://clinicaltrials.gov/study/NCT04553432
Mechanism of Benefit
Omnigen® + OmniLenz® works by:
1. Providing a biological scaffold for epithelial migration
2. Reducing inflammation and fibrosis through retained growth-factor activity
3. Enabling outpatient application, reducing need for OR, sutures, or theatre logistics
https://www.mdpi.com/1648-9144/60/6/985

Real-World Case Highlight
In a rare case of Enterococcus faecalis keratitis in a neurotrophic cornea, topical antibiotics plus a dry amniotic membrane patch resulted in full corneal healing at 11 weeks — highlighting the role of amniotic-membrane therapy in complex, high-risk infections.
Pubmed case Report
Practical Advantages for Clinicians
• Outpatient: no theatre required
• Shelf-stable: room-temperature storage cuts logistics
• Broad applicability: neurotrophic, infectious, post-surgical, exposure-related PEDs
• Suture-free: greater patient comfort and faster pathway
Nuvision Info
Conclusion
For ophthalmologists facing challenging ocular surface disease, Omnigen® + OmniLenz® by NuVision Biotherapies represents a major step in making amniotic-membrane therapy accessible, outpatient-friendly, and clinically effective. By combining biological efficacy with practical delivery, this innovation supports faster patient recovery, fewer surgical interventions and improved visual outcomes.
Contact us: info@aksia-health.com
Follow AKSIA Healthcare for more scientific updates and insights